Calcium chloride (CaCl2) is a chemical compound that is widely used in various industries. It is known as a mineral salt with moisture-absorbing properties and high solubility. In this article, we will examine the methods for preparing calcium chloride and explain the different steps of this process in detail.
What is calcium chloride?
Calcium chloride is an ionic compound composed of calcium and chlorine. It exists in nature in hydrated and non-hydrated forms and is used in various industries such as food, chemical, construction and even in road deicing. Calcium chloride absorbs moisture and is used in the production of some chemicals and pharmaceuticals.
Sources of calcium chloride
Calcium chloride occurs naturally in some mineral sources. It can be extracted from minerals or produced through chemical processes. One of the main sources of calcium chloride is seawater and salt lakes, which have a high concentration of mineral salts.

methods for preparing calcium chloride
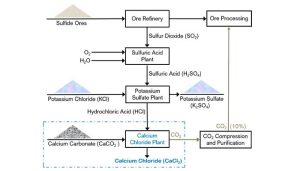
Calcium chloride is produced in three ways. The method of preparation of calcium chloride is given below:
Extraction from natural sources
The first method of preparing calcium chloride is to extract it from natural sources. This method has the following steps:
Mining of minerals: Minerals containing calcium and chlorine are extracted.
Washing and purification: The extracted rocks are washed and purified to remove impurities.
Dissolving in water: The purified rocks are dissolved in water to obtain a calcium chloride solution.
Evaporation: The resulting solution is evaporated to obtain crystalline calcium chloride.
Production from chemical reactions
The use of chemical reactions is another method for preparing calcium chloride. This method involves the following steps:
Preparation of calcium hydroxide: Calcium hydroxide (Ca(OH)2) is obtained from the reaction of calcium with water.
Reaction with hydrochloric acid: Calcium hydroxide reacts with hydrochloric acid (HCl) to produce calcium chloride.
Purification and crystallization: The resulting calcium chloride solution is purified and then crystallized.
Production from other salts
Calcium chloride can also be produced from other salts such as calcium carbonate (CaCO3). This method involves the following steps:
Dissolving calcium carbonate in hydrochloric acid: Calcium carbonate reacts with hydrochloric acid to produce calcium chloride.
Purification and evaporation: The resulting solution is purified and then evaporated to obtain crystalline calcium chloride.
What are the uses of calcium chloride?
Calcium chloride is used in various industries due to its unique characteristics and properties. Some of the uses of this material include:
- It is used as a preservative and moisture absorber in the food industry.
- It is used in the construction industry as an additive in concrete to increase the setting speed.
- It is used as an antifreeze to prevent roads and passages from freezing.
- Calcium chloride is a raw material in the production of other chemical compounds.
Safety tips for preparing calcium chloride
When preparing calcium chloride, it is very important to follow safety tips. Some of these tips include:
- Use safety equipment such as gloves and safety glasses
- Work in a well-ventilated area
- Avoid direct contact with skin and eyes
Challenges in Calcium Chloride Production
The production of calcium chloride comes with challenges that can affect the quality and final cost of the product. One of the main challenges is the supply of quality and sustainable raw materials. Also, chemical processes can result in waste and environmental pollution that need to be managed.
Environmental Challenges
The production of calcium chloride can lead to greenhouse gas emissions and water pollution. For this reason, companies must look for more sustainable and less harmful methods of producing this material. The use of modern technologies reduces environmental impacts.
Increasing Demand
Given the growing trend of various industries and the need for quality chemicals, the demand for calcium chloride is expected to increase in the future. This material will play an important role in sustainable development in the food, construction and chemical industries.
Economic Impacts of Calcium Chloride Production
The production of calcium chloride not only affects various industries, but also contributes to the overall economy of countries. Given the wide applications of this material, creating job opportunities and increasing exports can help improve the economic situation.
Job Creation
The production of calcium chloride can lead to the creation of new jobs in various fields, including the extraction, production, distribution and sale of this material.
Impact on Exports
Countries that have the ability to produce high-quality calcium chloride can access global markets and increase their exports.
Summary
Calcium chloride is an important chemical compound that has many applications in various industries. The methods for preparing calcium chloride includes extraction from natural sources and production through chemical reactions. Given the wide applications of calcium chloride, understanding the preparation methods and safety tips related to it is very important for industrialists and researchers. For information on the price of edible and industrial calcium chloride, call 03137779443.




No comment